- Review
- Open access
- Published:
Cystic fibrosis transmembrane conductance regulator (CFTR): beyond cystic fibrosis
Egyptian Journal of Medical Human Genetics volume 23, Article number: 94 (2022)
Abstract
Background
The cystic fibrosis transmembrane conductance regulator (CFTR) gene has been traditionally linked to cystic fibrosis (CF) inheritance in an autosomal recessive manner. Advances in molecular biology and genetics have expanded our understanding of the CFTR gene and its encoding products expressed in different tissues.
Aim
The study’s aim consists of reviewing the different pathological CF phenotypes using the existing literature. We know that alterations of the CFTR protein’s structure may result in different pathological phenotypes.
Methods
Open sources such as PubMed and Science Direct databases have been used for this review. We focused our selection on articles published within the last 15 years. Critical terms related to the CFTR protein have been used: “CFTR AND cancer,” “CFTR AND celiac disease,” “CFTR AND pancreatitis,” “children,” “adults,” “genotype,” “phenotype,” “correlation,” “mutation,” “CFTR,” “diseases,” “disorders,” and “no cystic fibrosis.”
Results
We analyzed 1,115 abstracts in total. Moreover, only 189 were suitable for the topic. We focused on the role of CFTR in cancer, gastrointestinal disorders, respiratory diseases, reproductive system, and systemic hypertension.
Conclusions
Mutations in CFTR gene are often associated with CF. In this review, we highlighted the broad spectrum of alterations reported for this gene, which may be involved in the pathogenesis of other diseases. The importance of these new insights in the role of CFTR relies on the possibility of considering this protein/gene as a novel therapeutic target for CF- and CFTR-related diseases.
Introduction
The gene that encodes the human cystic fibrosis transmembrane conductance regulator (CFTR) protein is located on the long arm of chromosome 7. It encodes for a membrane protein, specifically the ATP-binding cassette transporter-class ion channel protein that conducts chloride and thiocyanate ions across epithelial cell membranes. Mutations in the CFTR gene result in cystic fibrosis (CF), an autosomal recessive disorder. Since its discovery in 1989, over 2,300 variations are reported for the CFTR gene [1, 2].
Genetic testing availability and accessibility have broadened the spectrum of genotypes related to CFTR and the genotype–phenotype correlation, especially for milder phenotypes [3,4,5]. CFTR is expressed in different tissues and organs, mainly lung, gastrointestinal, and reproductive systems. Mutations affecting chloride ion channel function might lead to dysregulation of epithelial fluid transport in the lung and pancreas and affect other organs [6, 7].
New evidence has, recently, reconsidered the role of CFTR in diseases other than cystic fibrosis [8]. CFTR dysfunctions have been reported in high-heavy diseases such as cancer, celiac disease, and chronic obstructive pulmonary disease (COPD) [8]. Unlike CF, the suggested underlying pathogenic mechanisms include heterozygous mutations and an acquired dysfunction, such as epigenetic mechanisms [8]. This study reviews the existing literature on the different pathological phenotypes other than CF caused by alterations to the CFTR protein.
Methods
Research strategy
Open sources such as PubMed and Science Direct databases have been used for this review. We focused our selection on articles published within the last 15 years. Critical terms related to the CFTR protein have been used: “CFTR AND cancer,” “CFTR AND celiac disease,” “CFTR AND pancreatitis,” “children,” “adults,” “genotype,” “phenotype,” “correlation,” “mutation,” “CFTR,” “diseases,” “disorders,” and “no cystic fibrosis.”
Study selection
The following inclusion criteria have been considered in the article selection: English language, publication in peer-reviewed journals, published since 2006. All the articles irrelevant to the investigated issue have been excluded by title, abstract, or full text. Articles concerning cystic fibrosis were excluded. The studies containing inclusion criteria in the abstract have been considered for clarification. The selection has been extended to article’s references with similar inclusion criteria. A final number of 189 of 1,115 abstracts have satisfied the inclusion criteria.
CFTR and cancer
A growing number of studies have recently highlighted a correlation between mutations in the CFTR gene and different types of cancers [9–36, Table 1]. The role of CFTR in cancer seems to be variable according to the neoplasm, probably due to the influence and interaction with different tissue microenvironments [9].
A large population-based study on around 500,000 individuals assessed the association between CFTR mutation carriers, specifically F508del, and the risk of 54 types of cancers using the United Kingdom Biobank data [10]. Compared to non-cancer subjects, a significantly higher CFTR F508del mutation rate was found in individuals affected by colorectal ((OR 1.17 (95% CI 1.02–1.32, p = 0.02)), gallbladder and biliary tract ((OR 1.92 (95% CI 1.20–2.91, p = 0.004)), thyroid cancer ((OR 1.47 (95% CI 0.99–2.08, p = 0.04)), and non-Hodgkin's lymphoma ((OR 1.32 (95% CI 1.04–1.65, p = 0.02)) [10], and remained significantly high after multivariable analysis. Overall, lung cancer risk was reduced; however, in a similar study on Danish CFTR F508del, an increased risk of lung cancer was observed ((OR 1.52 (95% CI 1.12–2.08, p = 0.008)) [11]. No significant association between mutations and pancreatic cancer ((OR 1.2 (95% CI 0.85–1.64)) was observed [10]. This was consistent with Schubert et al. about pancreatic cancer, though they had a smaller study of 31 different CFTR mutations [12]. Nevertheless, a meta-analysis identified a modest but significantly increased risk of pancreatic cancer in four of the 13 CFTR mutation carriers (OR 1.41, 95% CI 1.07–1.84, p = 0.013, [mutations: F508del, W1282X, ΔI507, S549R]) [13]. Thus, this difference might be attributable to the spectrum of mutations analyzed.
The role of CFTR in cancer pathogenesis is not limited to gene mutations, but also through epigenetic modifications [14]. It has been suggested that the expression of CFTR was downregulated in patients affected by esophageal cancer (p < 0.05). Indeed, CFTR overexpression inhibited the growth and migration of esophageal cancer cells by downregulating protein expression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (p < 0.05). Conversely, CFTR silencing led to an increase in NF-κB-p65 and -p50 expression and increased tumor invasion, and growth in mice. [15].
Similarly, in colorectal cancer, CFTR expression was downregulated compared to normal tissues (p = 0.034), possibly due to the CFTR promoter's methylation. Interestingly, CFTR overexpression acts as a tumor suppressor and reduces cell migration and invasion [16], possibly through increased Wnt/beta-catenin signaling evidenced in CFTR knock-out mice, though this data remains to be verified [17]. Liu et al. found that knocked down CFTR colorectal cancer cells reduced heat shock protein 90 (HSP-90) expression (p < 0.01), leading to altered AKT and extracellular-regulated kinase (ERK) signaling pathways. Reduced AKT ERK signaling pathways determined mitochondrial dysfunction by inhibiting the Bcl-2 family proteins involved in apoptosis [18].
Hypermethylation of the CFTR promoter is observed in head and neck cancer and non-small cell lung, bladder, hepatic/hepatocellular, and breast cancer [19,20,21,22,23]. In the latter, CFTR silencing may lead to loss of E-cadherin, which assists in cell adhesion. The loss of E-cadherin correlates with invasion and metastasis [20, 24].
CFTR is expressed in the epithelial cells along the female reproductive tract [25]. CFTR overexpression in ovarian cancer is positively associated with severe cancers, at an advanced stage, and have a higher degree of malignancy, based on 112 tissue samples (83 epithelial ovarian cancer) (p < 0.05). The authors suggested a possible interaction with intracellular c-Src signaling pathways involved in cellular growth [26]. A similar observation concerning CFTR overexpression was reported in cervical cancer (p < 0.01), where it was associated with poorer prognosis, stage and metastasis. [27]. However, a more recent study contradicted these results, reporting that CFTR overexpression mediated the inhibition of the NF-κB p65 signaling pathway and reduced cell proliferation and tumor invasion [28]. In endometrial carcinoma, increased CFTR mRNA expression was identified (p < 0.05). Nevertheless, inhibition of CFTR with the CFTR inhibitor 172 promoted cell proliferation through reduced micro-RNA (miR)-125b, which acts as a tumor suppressor, decreasing the expression of matrix metalloproteinase 11 (MMP11), and vascular endothelial growth factor (VEGF) −A (p < 0.05) [29]. Further, high levels of urokinase plasminogen activator (uPA) are observed in prostate cancer, which leads to cell proliferation. Interestingly, a high association between CFTR expression and miR-193b expression was identified in those with prostate cancer. A reduction in CFTR expression leads to a reduction in miR-193A, with subsequent lack of inhibition of its target, the uPA [30]. Thus, these findings provide evidence for the many different interactions between CFTR and different signaling pathways, which goes beyond the original role of CFTR as an ion channel only.
The importance of detailed genetic characterization in cancer might have implications for diagnosis, prognosis, and treatment. Tu et al. identified CFTR downregulation in 225 cases of nasopharyngeal cancer. After multivariate analysis, CFTR downregulation resulted as an independent prognostic factor (p = 0.003), correlating with advanced cancer stages (p = 0.026), increased metastasis (p < 0.001), and poor prognosis (p < 0.01) [31]. Although lower CFTR expression was associated with a poor prognosis in 153 patients with glioblastoma (p = 0.04) [32], increased CFTR expression was also not beneficial to prostate cancer prognosis because it correlated with chemotherapy resistance [33]. However, Xie et al. reported that CFTR overexpression, whose was downregulated in prostate cancer by hypermethylation, suppressed prostate cancer progression in vitro and in vivo [30]. The emerging role of CFTR in cancer represents an intriguing potential therapeutic target. Indeed, like Forskolin and Cact-A1, CFTR activators demonstrated an antiproliferative effect on glioblastoma by significantly reducing Ki67 positive cells (p < 0.05) [32]. CFTR activation may decrease phosphorylation of Janus kinase 2/signal transducer and activator of transcription-3 (JAK2/STAT3) signaling pathway. This effect was attenuated by CFTR-inh172 (p < 0.05) [32, 33]. CFTR overexpression exerted a similar influence on lung adenocarcinoma Ki67 positive cells, as well as on cells’ invasion and migration. CFTR overexpression was also effective to inhibit clonogencity induced by nicotine exposure (p < 0.01) [34].
In contrast to this, in diseases where the Philadelphia chromosome is present, leading to T-cell acute lymphoblastic leukemia, CFTR expression was higher than normal controls (p < 0.001), Philadelphia chromosome negative acute lymphoblastic leukemia and chronic myeloid leukemia cells) (p < 0.01). Further, using the CFTR inhibitor, CFTR-inh172, inhibited T-cell acute lymphoblastic leukemia growth. The underlying mechanism of cell proliferation in this subtype of leukemia involved activating the p-BCR-ABL and Wnt/β-catenin signaling pathway. This leads to constant CFTR activation, which mediated inhibition of protein phosphatase 2A (PP2A) antitumoral activity [35, 36].
In summary, studies on cancer have overcome the conception of CFTR as a mere ion channel and transporter but showing pleiotropic interactions with proteins from different intracellular signaling pathways. The disruption of these pathways is from the consequence of gene mutations and the result of epigenetic modifications.
CFTR and gastrointestinal disorders
The most common gastrointestinal disorder associated with CFTR mutations is pancreatic insufficiency and pancreatitis in the setting of CF [37]. However, a fourfold increased risk of CFTR mutation carriers in idiopathic chronic pancreatitis (ICP) sufferers also has emerged, though the small sample size (OR 4.3, 95% CI 2.1–8.7, p = 0.0002). Further, Cohn extended the analysis to three previous studies. Overall, 14 CFTR mutation carriers out of 155 individuals with ICP were found, and the OR was 2.9 (1.7–4.9, p < 0.0001) [38]. The p.R75Q CFTR heterozygous variant is associated with ICP (OR = 3.43, 95% CI 1.7–6.8). The risk for ICP was much higher with the serine peptidase inhibitor (SPINK) 1 heterozygous variant (OR = 62.5, 95% CI 16.6–95.4). Other CFTR variants, except for F508del, became significant only when inherited together with SPINK1 variants, which may be due to CFTR impaired HCO3− conductance and not chloride conductance in p.R75Q carriers. Overall, CFTR carriers, including CF-causing variants, had an OR of 7.4 (95% CI 2.3–18.5) of those with ICP [39]. Two previous review study partially reconsidered these results, highlighting the predominant role of SPINK1 in those with ICP when non-CF-causing variants, such as p.R75Q, were detected. Their rate was not significantly different compared to controls carrying SPINK1 variants [40, 41].
Beyond CFTR, the most common genes associated with ICP were the cationic trypsinogen gene (PRSS1), SPINK1, and CTRC gene. In a Chinese cohort of 715 individuals with ICP, isolated CFTR variants were not significantly associated with ICP compared with 1,196 controls (p > 0.05) [42]. Therefore, although the association between CFTR and pancreatitis has been widely investigated, there are still contradictory results due to the complex genetic background and interactions between environmental factors.
Other gastrointestinal disorders should be added within the spectrum of the CFTR gene mutations and dysfunctions. These include secretory diarrheas, altered bile acid homeostasis, primary sclerosing cholangitis, and celiac disease [43]. CFTR targets bacterial enterotoxins on the intestinal epithelial cell membrane, which induce cAMP- or cGMP-mediated protracted activation of CFTR, leading to subsequent water loss and dehydration, typical features of secretory diarrheas. Thus, CFTR inhibitors, like CFTR-inh172, could represent a potential target therapy in secretory diarrheas. However, some limitations include the difficult localization of CFTR in the intestinal crypts and other ion channels' involvement in the pathogenesis of secretory diarrheas [43, 44].
Another organ affected by CFTR dysfunction is the gallbladder, as evidenced in CFTR knock-out mice, which showed impaired gallbladder emptying (p < 0.05), probably due to vasoactive intestinal peptide (VIP) overexpression which acts as a myorelaxant on gallbladder, and bile acid homeostasis [44]. Primary sclerosing cholangitis (PSC) is a chronic cholestasis condition associated with biliary inflammation, obliteration, and fibrosis [45]. Though PSC pathogenesis has been associated with specific HLAs (HLA-DRB1*1501-DQB1*0602, HLA-DRB1*1301-DQB1*0603, and HLA-A1-B8-DRB1*0301-DQB1*0201) and often with inflammatory bowel disease, a role for CFTR in PSC development has been suggested [46, 47]. Sheth et al. reported a significant increase in heterozygous CFTR variants in seven out of 19 (37%, 95% CI: 16–62%) individuals affected by PSC compared to inflammatory bowel disease and primary biliary cirrhosis patients (p < 0.02) [47]. In a cohort of 32 patients with PSC who underwent next-generation sequencing, six had CFTR disease causing mutations on one allele (OR = 6.1–95% CI 2.2–16.7, p = 0.002), and 19 carried at least one CFTR polymorphism. However, six had abnormal, and 21 had intermediate sweat tests associated with CF-like phenotype [48]. Previous studies questioned the relationship between CFTR and PSC, which did not find significant differences between patients with or without PSC and the prevalence of CFTR mutations. Nevertheless, these studies may be biased by the limited number of modifications analyzed [48, 49].
Celiac disease (CD) is strictly associated with HLA DQ2/DQ8 predisposition [50]. The evidence that CF patients reported having a significantly higher CD incidence (p = 0.0007) has led to an investigation of CFTR's function in patients with CD [51, 52]. Interestingly, Villella et al. revealed new insights in such a disease's pathogenesis, suggesting a CFTR role as a gluten target [53]. In previous studies, CFTR has been identified as a regulator of proteostasis, an essential cytoprotective mechanism to remove misfolded or polyubiquitylated proteins. Its dysfunction was associated with lung inflammation due to autophagy inhibition, mediated by tissue transglutaminase 2 (TGM2), a key enzyme involved in CD [54]. Villella et al. showed that alpha-gliadin-derived LGQQQPFPPQQPY peptide (P31–43) inhibits the ATPase function of CFTR by binding with the nucleotide-binding domain-1 (NBD1) of CFTR on intestinal epithelial cells of mice with CD-predisposing HLA. NBD1 interaction with P31–43 happened only when it is in a closed conformation. This has consequences on downstream pathways, the tissue transglutaminase 2 (TGM2), and the autophagy protein Beclin-1 (BECN1). Indeed, TGM2 activation, in response to CFTR inactivation, led to reduced BECN1 with impaired proteostasis, determining a pro-inflammatory state. Therefore, TGM2, inhibiting NF-κB inhibitor alpha (NFKBIA), determined increased NFκB and inflammasome activation with IL-15 and IL-β production, respectively. These initiate an immune response against gliadin [53].
The previously mentioned interactions created a vicious cycle that amplifies and further worsen CFTR inhibition. Hence, Maiuri et al. introduced the definition of “infernal trio” regarding CFTR inhibition, TGM2 activation, and autophagy impairment. For example, TGM2 activation promoted CFTR crosslinking with P31-43, creating a trimolecular complex, which made CFTR inhibition irreversible [55]. In the light of this mechanism, and the affinity of P31-43 peptide to the closed conformation of CFTR, potentiators of CFTR may be a therapeutic option in celiac disease, by promoting CFTR channel opening. VX-770 (Ivacaftor), a CFTR potentiator, seemed to effectively reduce gliadin-induced inflammation in vitro, especially IL-15 production and NF-kB p65, making CFTR a potential new therapeutic target in CD. Furthermore, VX-770 also prevented gliadin mediated inhibition of CFTR and promoted a tolerogenic response in gluten-sensitive mice and cells from celiac patients [53].
Genistein, a phytoestrogen contained in soy, targets CFTR and acts as a channel gating potentiate. In the context of celiac disease, it was able to prevent P31-43 induced epithelial stress and inflammation, both in vitro and in vivo animal models [56].
Drugs targeting CFTR may find application in two other autoimmune diseases, idiopathic autoimmune pancreatitis and Sjogren’s syndrome, characterized by disrupted fluid secretion in pancreatic ducts and of saliva and lacrimal glands, respectively. Indeed, in Sjogren's syndrome mice model CFTR expression was reduced and treatment with VX-770 and C18 increased salivation, ductal fluid secretion, and reduced inflammation. Similar results were obtained in a pancreatic inflammation model. Furthermore, C18 restored CFTR expression in the ducts of salivary glands. Interestingly, this had also effects on acinar cells, where Aquaporin 5 expression was recovered [57]. Table 2 summarizes the main studies about CFTR and gastrointestinal disorders.
CFTR and the lungs
Recently, acquired CFTR dysfunctions was found to occur in highly prevalent diseases such as chronic obstructive pulmonary disease (COPD) with a chronic bronchitis phenotype [58]. Indeed, cigarette smoke exposure reduces CFTR expression and activity, both in vitro and in vivo, contributing to mucociliary clearance impairment. Interestingly, CFTR dysfunction was not limited to the lungs but was evidenced in other areas, suggesting a potential role in the onset of smoke-related complications such as Mellitus diabetes, male infertility, and idiopathic pancreatitis [59]. The hypothesized mechanism may be attributable to acrolein, which induces structural changes to the CFTR channel, or cadmium and arsenic [59,60,61]. As regards cadmium, it showed to reduce CFTR expression (p < 0.001), both in vitro and in vivo, in a dose- and time-dependent manner and CFTR channel activity [60]. A similar effect was described for arsenic through ubiqutin-mediated lysosomal degradation of CFTR and reduced chloride secretion in vitro (p < 0.05) [61]. In a double-blind, placebo-controlled study on 92 COPD patients, the CFTR potentiator Icenticaftor 300 mg effectively improved FEV1 after 28 days (mean 50 mL for pre-bronchodilator FEV1 and mean 63 mL for post-bronchodilator FEV1), while no significant improvement was seen in the lung clearance index [62].
In a population-based study, 2858 carriers of CFTR F508del were identified and had an increased risk of chronic bronchitis (HR 1.31, 95% CI 1.16–1.48), bronchiectasis (HR 1.88, 95% CI 1.03–3.45), and lung cancer (HR 1.52, 95% CI 1.12–2.08). However, this study's main limitation is the lack of investigations for mutations different from F508del, which may be responsible for compound heterozygosis [11].
Bronchial asthma is certainly one of the main chronic diseases of the respiratory system [63, 64]. Although the risk of asthma was not higher than the general population, a meta-analysis of 15 studies (2.113 asthma cases and 13.457 controls) on CFTR mutations carriers, which contained 34 different pathogenetic variants, reported an increased risk of asthma (OR 1.61, 95% CI 1.18–2.21) [65]. Table 3 summarizes the main studies about CFTR and lung disorders.
CFTR and the reproductive system
Congenital bilateral absence of the vas deferens (CBAVD) accounts for approximately 3% of infertility cases. Because almost all infertile CF males exhibit CBAVD, it is widely considered an atypical form of CF and a CFTR-related disorder [7]. Interestingly, a meta-analysis on 38 studies found that 28% (95% CI 24–32%) of individuals with CBAVD carried only one CFTR mutation. However, there is a considerable risk of bias because of the included study's heterogeneity (Egger’s test p = 0.874), which might not have investigated less common CFTR [66]. Recently, heterozygous copy number variants of CFTR have also been suggested to play a role in the pathogenesis of CBAVD, reported to affect five (1.9%) of 263 Chinese affected individuals. Among these, four out of five carried a CFTR partial deletion [67].
Regarding the female reproductive system, in polycystic ovarian syndrome (PCOS), CFTR and aromatase expression was downregulated in granulosa cells, both in rat model and human, compared with non-PCOS women (p < 0.05). Hence, Chen et al. found that CFTR enhanced follicle-stimulating hormone (FSH) mediated aromatase expression through HCO3-induced cAMP response element-binding protein (CREB) phosphorylation [68]. In a more recent study, the CFTR/HCO3-/sAC signaling pattern was further elucidated, contributing to MAPK/ERK downstream pathways of cell proliferation. Therefore, defective CFTR decreased granulosa cell proliferation and contributed to altered follicle formation, typical of PCOS [69]. Table 4 summarizes the main studies about CFTR and reproductive system disorders.
CFTR and cardiovascular system
CFTR seems to be involved in blood pressure regulation. CFTR knock-out mice developed higher mean blood pressure compared with controls (p < 0.05). This evidence was supported by CFTR downregulation in a model of induced hypertension, where a high fructose and salt diet reduced With-No-Lysine K (WNK) kinase expression in arteries and subsequently CFTR expression, leading to increased vascular constriction. This was further supported by the evidence that CFTR knock-out mice fed with high fructose and salt dose did not show increased mean blood pressure (p < 0.05). [70]. However, further studies are needed to confirm these preliminary data.
Conclusions
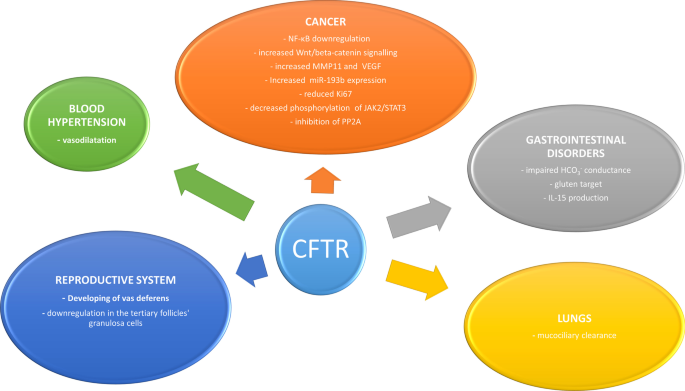
Mutations in CFTR gene are often associated with CF. In this review, we highlighted the broad spectrum of alterations reported for this gene, which may be involved in the pathogenesis of other diseases [Fig. 1]. The extensive application of genetic testing provides evidence to link the CFTR gene mutation with different conditions and characterize their genotype-phenotypes. The importance of these new insights in the role of CFTR relies on the possibility of making it a novel therapeutic target. In this context, results obtained by the new CFTR modulators in CF are promising, highlighting their ability to modify the disease course. Therefore, they might improve treatment of high burden diseases through precision medicine [71]. However, despite the ubiquitous role of CFTR is various organ systems, there is still a dearth of CFTR-based therapeutics. Some of the reasons might be unstable pharmacokinetics, cross-talk with other cellular proteins, and sampling issues for clinical trials. These points have to be considered while developing CFTR-based therapeutic interventions.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- AKT:
-
Protein kinase B
- ATP:
-
Adenosine triphosphate
- BECN1:
-
Beclin-1
- cAMP:
-
Cyclic adenosine monophosphate
- CBAVD:
-
Congenital bilateral absence of the vas deferens
- CD:
-
Celiac disease
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- cGMP:
-
Cyclic guanosine monophosphate
- COPD:
-
Chronic obstructive pulmonary disease
- CREB:
-
cAMP response element-binding protein
- CTRC:
-
Chymotrypsinogen C
- ERK:
-
Extracellular-regulated kinase
- FEV1:
-
Forced expiratory volume in 1 s
- FSH:
-
Follicle-stimulating hormone
- HSP-90:
-
Heat shock protein 90
- ICP:
-
Idiopathic chronic pancreatitis
- JAK2:
-
Janus kinase 2
- miR:
-
Reduced micro-RNA
- MMP11:
-
Matrix metalloproteinase 11
- NBD1:
-
Nucleotide-binding domain-1
- NFKBIA:
-
NF-κB inhibitor alpha
- NF-Κb:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OR:
-
Odds ratio
- PCOS:
-
Polycystic ovarian syndrome
- PP2A:
-
Protein phosphatase 2A
- PRSS1:
-
Cationic trypsinogen gene
- PSC:
-
Primary sclerosing cholangitis
- SPINK:
-
Serine peptidase inhibitor
- STAT3:
-
Signal transducer and activator of transcription-3
- TGM2:
-
Tissue transglutaminase 2
- Upa:
-
Urokinase plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
- VIP:
-
Vasoactive intestinal peptide
- WNK:
-
With-no-lysine K
References
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245(4922):1066-73. https://doi.org/10.1126/science.2475911. Erratum in: Science 1989 Sep 29;245(4925):1437
http://www.genet.sickkids.on.ca/ Accessed on 21th January, 2021
Parisi GF, Cutello S, Di Dio G, Rotolo N, La Rosa M, Leonardi S (2013) Phenotypic expression of the p.Leu1077Pro CFTR mutation in Sicilian cystic fibrosis patients. BMC Res Notes 6:461. https://doi.org/10.1186/1756-0500-6-461
Leonardi S, Parisi GF, Capizzi A, Manti S, Cuppari C, Scuderi MG, Rotolo N, Lanzafame A, Musumeci M, Salpietro C (2016) YKL-40 as marker of severe lung disease in cystic fibrosis patients. J Cyst Fibros 15(5):583–586. https://doi.org/10.1016/j.jcf.2015.12.020
Spicuzza L, Parisi GF, Tardino L, Ciancio N, Nenna R, Midulla F, Leonardi S (2018) Exhaled markers of antioxidant activity and oxidative stress in stable cystic fibrosis patients with moderate lung disease. J Breath Res 12(2):026010. https://doi.org/10.1088/1752-7163/aa9b39
Parisi GF, Portale A, Papale M, Tardino L, Rotolo N, Licari A, Leonardi S (2019) Successful treatment with omalizumab of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis: case reports and literature review. J Allergy Clin Immunol Pract 7(5):1636–1638. https://doi.org/10.1016/j.jaip.2019.01.056
Bombieri C, Claustres M, De Boeck K, Derichs N, Dodge J, Girodon E, Sermet I, Schwarz M, Tzetis M, Wilschanski M, Bareil C, Bilton D, Castellani C, Cuppens H, Cutting GR, Drevínek P, Farrell P, Elborn JS, Jarvi K, Kerem B, Kerem E, Knowles M, Macek M Jr, Munck A, Radojkovic D, Seia M, Sheppard DN, Southern KW, Stuhrmann M, Tullis E, Zielenski J, Pignatti PF, Ferec C (2011) Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros 10(Suppl 2):S86-102. https://doi.org/10.1016/S1569-1993(11)60014-3
Galluzzi L, Kroemer G (2019) Etiological involvement of CFTR in apparently unrelated human diseases. Mol Cell Oncol. https://doi.org/10.1080/23723556.2018.1558874
Zhang J, Wang Y, Jiang X, Chan HC (2018) Cystic fibrosis transmembrane conductance regulator-emerging regulator of cancer. Cell Mol Life Sci 75(10):1737–1756. https://doi.org/10.1007/s00018-018-2755-6
Shi Z, Wei J, Na R, Resurreccion WK, Zheng SL, Hulick PJ, Helfand BT, Talamonti MS, Xu J (2021) Cystic fibrosis F508del carriers and cancer risk: Results from the UK Biobank. Int J Cancer 148(7):1658–1664. https://doi.org/10.1002/ijc.33431
Çolak Y, Nordestgaard BG, Afzal S (2020) Morbidity and mortality in carriers of the cystic fibrosis mutation CFTR Phe508del in the general population. Eur Respir J. https://doi.org/10.1183/13993003.00558-2020
Schubert S, Traub F, Brakensiek K, von Kopylow K, Marohn B, Maelzer M, Gaedcke J, Kreipe H, Stuhrmann M (2014) CFTR, SPINK1, PRSS1, and CTRC mutations are not associated with pancreatic cancer in German patients. Pancreas 43(7):1078–1082. https://doi.org/10.1097/MPA.0000000000000166
Cazacu IM, Farkas N, Garami A, Balaskó M, Mosdósi B, Alizadeh H, Gyöngyi Z, Rakonczay Z Jr, Vigh É, Habon T, Czopf L, Lazarescu MA, Erőss B, Sahin-Tóth M, Hegyi P (2018) Pancreatitis-associated genes and pancreatic cancer risk: a systematic review and meta-analysis. Pancreas 47(9):1078–1086. https://doi.org/10.1097/MPA.0000000000001145
Esteller M (2008) Epigenetics in cancer. N Engl J Med. https://doi.org/10.1056/nejmra072067
Li W, Wang C, Peng X, Zhang H, Huang H, Liu H (2018) CFTR inhibits the invasion and growth of esophageal cancer cells by inhibiting the expression of NF-κB. Cell Biol Int 42(12):1680–1687. https://doi.org/10.1002/cbin.11069
Liu C, Song C, Li J, Sun Q (2020) CFTR Functions as a tumor suppressor and is regulated by DNA methylation in colorectal cancer. Cancer Manag Res 12:4261–4270. https://doi.org/10.2147/CMAR.S248539
Than BL, Linnekamp JF, Starr TK, Largaespada DA, Rod A, Zhang Y, Bruner V, Abrahante J, Schumann A, Luczak T, Walter J, Niemczyk A, O'Sullivan MG, Medema JP, Fijneman RJ, Meijer GA, Van den Broek E, Hodges CA, Scott PM, Vermeulen L, Cormier RT. (2016) CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 35 (32):4179-87. https://doi.org/10.1038/onc.2015.483 Erratum in: Oncogene. 2017, 36(24):3504
Liu K, Jin H, Guo Y, Liu Y, Wan Y, Zhao P, Zhou Z, Wang J, Wang M, Zou C, Wu W, Cheng Z, Dai Y (2019) CFTR interacts with Hsp90 and regulates the phosphorylation of AKT and ERK1/2 in colorectal cancer cells. FEBS Open Bio. 9(6):1119–1127. https://doi.org/10.1002/2211-5463.12641
Shin Y, Kim M, Won J, Kim J, Oh SB, Lee JH, Park K (2020) Epigenetic modification of CFTR in head and neck cancer. J Clin Med 9(3):734. https://doi.org/10.3390/jcm9030734
Liu K, Dong F, Gao H, Guo Y, Li H, Yang F, Zhao P, Dai Y, Wang J, Zhou W, Zou C (2020) Promoter hypermethylation of the CFTR gene as a novel diagnostic and prognostic marker of breast cancer. Cell Biol Int 44(2):603–609. https://doi.org/10.1002/cbin.11260
Son JW, Kim YJ, Cho HM, Lee SY, Lee SM, Kang JK, Lee JU, Lee YM, Kwon SJ, Choi E, Na MJ, Park JY, Kim DS (2011) Promoter hypermethylation of the CFTR gene and clinical/pathological features associated with non-small cell lung cancer. Respirology 16(8):1203–1209. https://doi.org/10.1111/j.1440-1843.2011.01994.x
Yu J, Zhu T, Wang Z, Zhang H, Qian Z, Xu H, Gao B, Wang W, Gu L, Meng J, Wang J, Feng X, Li Y, Yao X, Zhu J (2007) A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin Cancer Res 13(24):7296–7304. https://doi.org/10.1158/1078-0432.CCR-07-0861
Moribe T, Iizuka N, Miura T, Kimura N, Tamatsukuri S, Ishitsuka H, Hamamoto Y, Sakamoto K, Tamesa T, Oka M (2009) Methylation of multiple genes as molecular markers for diagnosis of a small, well-differentiated hepatocellular carcinoma. Int J Cancer 125(2):388–397. https://doi.org/10.1002/ijc.24394
Zhang JT, Jiang XH, Xie C, Cheng H, Da Dong J, Wang Y, Fok KL, Zhang XH, Sun TT, Tsang LL, Chen H, Sun XJ, Chung YW, Cai ZM, Jiang WG, Chan HC (2013) Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim Biophys Acta 1833(12):2961–2969. https://doi.org/10.1016/j.bbamcr.2013.07.021
Tizzano EF, Silver MM, Chitayat D, Benichou JC, Buchwald M (1994) Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues: clues for the infertility in patients with cystic fibrosis. Am J Pathol 144(5):906
Xu J, Yong M, Li J, Dong X, Yu T, Fu X, Hu L (2015) High level of CFTR expression is associated with tumor aggression and knockdown of CFTR suppresses proliferation of ovarian cancer in vitro and in vivo. Oncol Rep 33(5):2227–2234. https://doi.org/10.3892/or.2015.3829
Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC, Zhang Y, Hu L (2012) Overexpression of cystic fibrosis transmembrane conductance regulator (CFTR) is associated with human cervical cancer malignancy, progression and prognosis. Gynecol Oncol 125(2):470–476. https://doi.org/10.1016/j.ygyno.2012.02.015
Wu Z, Li J, Zhang Y, Hu L, Peng X (2020) CFTR regulates the proliferation, migration and invasion of cervical cancer cells by inhibiting the NF-κB signalling pathway. Cancer Manag Res. https://doi.org/10.2147/CMAR.S252296
Xia X, Wang J, Liu Y, Yue M (2017) Lower cystic fibrosis transmembrane conductance regulator (CFTR) promotes the proliferation and migration of endometrial carcinoma. Med Sci Monit. https://doi.org/10.12659/MSM.899341
Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, Yu MK, Zhang XH, Chung YW, Ye L, Zhao MY, Guo JH, Xiao ZJ, Lan HY, Ng CF, Lau KM, Cai ZM, Jiang WG, Chan HC (2013) CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 32(18):2282-91-2291.e1–7. https://doi.org/10.1038/onc.2012.251
Tu Z, Chen Q, Zhang JT, Jiang X, Xia Y, Chan HC (2016) CFTR is a potential marker for nasopharyngeal carcinoma prognosis and metastasis. Oncotarget. https://doi.org/10.18632/oncotarget.12762
Zhong X, Chen HQ, Yang XL, Wang Q, Chen W, Li C (2019) CFTR activation suppresses glioblastoma cell proliferation, migration and invasion. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2018.12.080
Zhu Q, Li H, Liu Y, Jiang L (2017) Knockdown of CFTR enhances sensitivity of prostate cancer cells to cisplatin via inhibition of autophagy. Neoplasma. https://doi.org/10.4149/neo_2017_508
Li H, Ma N, Wang J, Wang Y, Yuan C, Wu J, Luo M, Yang J, Chen J, Shi J, Liu X (2018) Nicotine induces progressive properties of lung adenocarcinoma A549 cells by inhibiting cystic fibrosis transmembrane conductance regulator (CFTR) expression and plasma membrane localization. Technol Cancer Res Treat 1(17):1533033818809984. https://doi.org/10.1177/1533033818809984
Yang X, Yan T, Gong Y, Liu X, Sun H, Xu W, Wang C, Naren D, Zheng Y (2017) High CFTR expression in Philadelphia chromosome-positive acute leukemia protects and maintains continuous activation of BCR-ABL and related signaling pathways in combination with PP2A. Oncotarget 8(15):24437–24448. https://doi.org/10.18632/oncotarget.15510
Liu M, Liao H, Chen Y, Lin Z, Liu Y, Zhang X, Chan HC, Sun H (2019) Treatment of human T-cell acute lymphoblastic leukemia cells with CFTR inhibitor CFTRinh-172. Leuk Res 86:106225. https://doi.org/10.1016/j.leukres.2019.106225
Parisi GF, Papale M, Rotolo N, Aloisio D, Tardino L, Scuderi MG, Di Benedetto V, Nenna R, Midulla F, Leonardi S (2017) Severe disease in cystic fibrosis and fecal calprotectin levels. Immunobiology 222(3):582–586. https://doi.org/10.1016/j.imbio.2016.11.005
Cohn JA, Neoptolemos JP, Feng J, Yan J, Jiang Z, Greenhalf W, McFaul C, Mountford R, Sommer SS (2005) Increased risk of idiopathic chronic pancreatitis in cystic fibrosis carriers. Hum Mutat 26(4):303–307. https://doi.org/10.1002/humu.20232
Schneider A, Larusch J, Sun X, Aloe A, Lamb J, Hawes R, Cotton P, Brand RE, Anderson MA, Money ME, Banks PA, Lewis MD, Baillie J, Sherman S, Disario J, Burton FR, Gardner TB, Amann ST, Gelrud A, George R, Rockacy MJ, Kassabian S, Martinson J, Slivka A, Yadav D, Oruc N, Barmada MM, Frizzell R, Whitcomb DC (2011) Combined bicarbonate conductance-impairing variants in CFTR and SPINK1 variants are associated with chronic pancreatitis in patients without cystic fibrosis. Gastroenterologyb 140(1):162–71. https://doi.org/10.1053/j.gastro.2010.10.045
Rosendahl J, Landt O, Bernadova J, Kovacs P, Teich N, Bödeker H, Keim V, Ruffert C, Mössner J, Kage A, Stumvoll M, Groneberg D, Krüger R, Luck W, Treiber M, Becker M, Witt H (2013) CFTR, SPINK1, CTRC and PRSS1 variants in chronic pancreatitis: is the role of mutated CFTR overestimated? Gut 62(4):582–592. https://doi.org/10.1136/gutjnl-2011-300645
Raphael KL, Willingham FF (2016) Hereditary pancreatitis: current perspectives. Clin Exp Gastroenterol 9:197–207. https://doi.org/10.2147/CEG.S84358
Zou WB, Tang XY, Zhou DZ, Qian YY, Hu LH, Yu FF, Yu D, Wu H, Deng SJ, Lin JH, Zhao AJ, Zhao ZH, Wu HY, Zhu JH, Qian W, Wang L, Xin L, Wang MJ, Wang LJ, Fang X, He L, Masson E, Cooper DN, Férec C, Li ZS, Chen JM, Liao Z (2018) SPINK1, PRSS1, CTRC, and CFTR genotypes influence disease onset and clinical outcomes in chronic pancreatitis. Clin Transl Gastroenterol 9(11):204. https://doi.org/10.1038/s41424-018-0069-5
de Jonge HR, Ardelean MC, Bijvelds MJC, Vergani P (2020) Strategies for cystic fibrosis transmembrane conductance regulator inhibition: from molecular mechanisms to treatment for secretory diarrhoeas. FEBS Lett 594(23):4085–4108. https://doi.org/10.1002/1873-3468.13971
Debray D, Rainteau D, Barbu V, Rouahi M, El Mourabit H, Lerondel S, Rey C, Humbert L, Wendum D, Cottart CH, Dawson P, Chignard N, Housset C (2012) Defects in gallbladder emptying and bile acid homeostasis in mice with cystic fibrosis transmembrane conductance regulator deficiencies. Gastroenterology 142(7):1581–91.e6. https://doi.org/10.1053/j.gastro.2012.02.033
Lazaridis KN, LaRusso NF (2016) Primary sclerosing cholangitis. N Engl J Med 375(12):1161–1170. https://doi.org/10.1056/NEJMra1506330
Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD (2013) Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. https://doi.org/10.1053/j.gastro.2013.06.052
Sheth S, Shea JC, Bishop MD, Chopra S, Regan MM, Malmberg E, Walker C, Ricci R, Tsui LC, Durie PR, Zielenski J, Freedman SD (2003) Increased prevalence of CFTR mutations and variants and decreased chloride secretion in primary sclerosing cholangitis. Hum Genet 113(3):286–292. https://doi.org/10.1007/s00439-003-0963-z
Werlin S, Scotet V, Uguen K, Audrezet MP, Cohen M, Yaakov Y, Safadi R, Ilan Y, Konikoff F, Galun E, Mizrahi M, Slae M, Sayag S, Cohen-Cymberknoh M, Wilschanski M, Ferec C (2018) Primary sclerosing cholangitis is associated with abnormalities in CFTR. J Cyst Fibros 17(5):666–671. https://doi.org/10.1016/j.jcf.2018.04.005
Gallegos-Orozco JF, Yurk EC, Wang N, Rakela J, Charlton MR, Cutting GR, Balan V (2005) Lack of association of common cystic fibrosis transmembrane conductance regulator gene mutations with primary sclerosing cholangitis. Am J Gastroenterol. 100(4):874–8. https://doi.org/10.1111/j.1572-0241.2005.41072.x
Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, Barbato M, Barbera C, Barera G, Bellantoni A, Castellano E, Guariso G, Limongelli MG, Pellegrino S, Polloni C, Ughi C, Zuin G, Fasano A, Catassi C (2014) SIGENP (Italian society of pediatric gastroenterology, hepatology, and nutrition) working group on weaning and CD risk introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 371(14):1295–303. https://doi.org/10.1056/NEJMoa1400697
Walkowiak J, Blask-Osipa A, Lisowska A, Oralewska B, Pogorzelski A, Cichy W, Sapiejka E, Kowalska M, Korzon M, Szaflarska-Popławska A (2010) Cystic fibrosis is a risk factor for celiac disease. Acta Biochim Pol 57(1):115–118
Lionetti E, Gatti S, Pulvirenti A, Catassi C (2015) Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol 29(3):365–379. https://doi.org/10.1016/j.bpg.2015.05.004
Villella VR, Venerando A, Cozza G, Esposito S, Ferrari E, Monzani R, Spinella MC, Oikonomou V, Renga G, Tosco A, Rossin F, Guido S, Silano M, Garaci E, Chao YK, Grimm C, Luciani A, Romani L, Piacentini M, Raia V, Kroemer G, Maiuri L (2019) A pathogenic role for cystic fibrosis transmembrane conductance regulator in celiac disease. EMBO J 38(2):100101. https://doi.org/10.15252/embj.2018100101
Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D’Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L (2010) Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12(9):863–875. https://doi.org/10.1038/ncb2090
Maiuri L, Villella VR, Piacentini M, Raia V, Kroemer G (2019) Defective proteostasis in celiac disease as a new therapeutic target. Cell Death Dis. https://doi.org/10.1038/s41419-019-1392-9
Esposito S, Villella VR, Ferrari E, Monzani R, Tosco A, Rossin F, D’Eletto M, Castaldo A, Luciani A, Silano M, Bona G, Marseglia GL, Romani L, Piacentini M, Raia V, Kroemer G, Maiuri L (2019) Genistein antagonizes gliadin-induced CFTR malfunction in models of celiac disease. Aging (Albany NY) 11(7):2003–2019. https://doi.org/10.18632/aging.101888
Zeng M, Szymczak M, Ahuja M, Zheng C, Yin H, Swaim W, Chiorini JA, Bridges RJ, Muallem S (2017) Restoration of CFTR activity in ducts rescues acinar cell function and reduces inflammation in pancreatic and salivary glands of mice. Gastroenterology 153(4):1148–1159. https://doi.org/10.1053/j.gastro.2017.06.011
Fernandez Fernandez E, De Santi C, De Rose V, Greene CM (2018) CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev Respir Med 12(6):483–492. https://doi.org/10.1080/17476348.2018
Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM (2013) Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 188(11):1321–1330. https://doi.org/10.1164/rccm.201304-0733OC
Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E (2010) Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol Sci 116(1):349–58. https://doi.org/10.1093/toxsci/kfq101
Bomberger JM, Coutermarsh BA, Barnaby RL, Stanton BA (2012) Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells. J Biol Chem. https://doi.org/10.1074/jbc.M111.338855
Rowe SM, Jones I, Dransfield MT, Haque N, Gleason S, Hayes KA, Kulmatycki K, Yates DP, Danahay H, Gosling M, Rowlands DJ, Grant SS (2020) Efficacy and safety of the CFTR potentiator icenticaftor (QBW251) in COPD: results from a phase 2 randomized trial. Int J Chron Obstruct Pulmon Dis 5(15):2399–2409. https://doi.org/10.2147/COPD.S257474
Licari A, Manti S, Castagnoli R, Parisi GF, Salpietro C, Leonardi S, Marseglia GL (2019) Targeted therapy for severe asthma in children and adolescents: current and future perspectives. Paediatr Drugs 21(4):215–237. https://doi.org/10.1007/s40272-019-00345-7
Leonardi S, Filippelli M, Lanzafame A, Parisi G, Mistrello G, Musumeci M, Torrisi V, Musumeci S, Cuppari C (2019) Serum YKL-40 in children with asthma. J Biol Regul Homeost Agents 29(21):114–9
Nielsen AO, Qayum S, Bouchelouche PN, Laursen LC, Dahl R, Dahl M (2016) Risk of asthma in heterozygous carriers for cystic fibrosis: a meta-analysis. J Cyst Fibros. https://doi.org/10.1016/j.jcf.2016.06.001
Yu J, Chen Z, Ni Y, Li Z (2012) CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum Reprod 27(1):25–35. https://doi.org/10.1093/humrep/der377
Ma C, Wang R, Li T, Li H, Wang B (2020) Analysis of CNVs of CFTR gene in Chinese Han population with CBAVD. Mol Genet Genomic Med. https://doi.org/10.1002/mgg3.1506
Chen H, Guo JH, Lu YC, Ding GL, Yu MK, Tsang LL, Fok KL, Liu XM, Zhang XH, Chung YW, Huang P, Huang H, Chan HC (2012) Impaired CFTR-dependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS. J Clin Endocrinol Metab 97(3):923–932. https://doi.org/10.1210/jc.2011-1363
Chen H, Guo JH, Zhang XH, Chan HC (2015) Defective CFTR-regulated granulosa cell proliferation in polycystic ovarian syndrome. Reproduction. https://doi.org/10.1530/REP-14-0368
Zhang Y-P, Ye LL, Yuan H, Duan DD (2020) CFTR plays an important role in the regulation of vascular resistance and high-fructose/salt-diet induced hypertension in mice. J Cyst Fibros Off J Eur Cyst Fibros Soc. https://doi.org/10.1016/j.jcf.2020.11.014
Dagenais RVE, Su VCH, Quon BS (2020) Real-world safety of CFTR modulators in the treatment of cystic fibrosis: a systematic review. J Clin Med 10(1):23. https://doi.org/10.3390/jcm10010023
Acknowledgements
Not applicable
Funding
The authors did not receive any funding for the research.
Author information
Authors and Affiliations
Contributions
GFP, FM, and AG wrote the manuscript; MP and SM performed the research and reviewed the manuscript; SL supervised the work; all authors have read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to disclose that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parisi, G.F., Mòllica, F., Giallongo, A. et al. Cystic fibrosis transmembrane conductance regulator (CFTR): beyond cystic fibrosis. Egypt J Med Hum Genet 23, 94 (2022). https://doi.org/10.1186/s43042-022-00308-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00308-7